The Approach
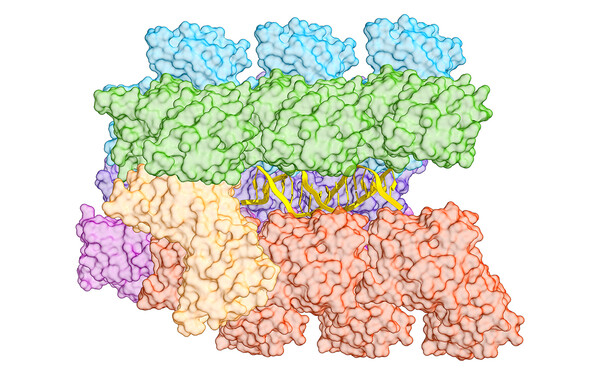
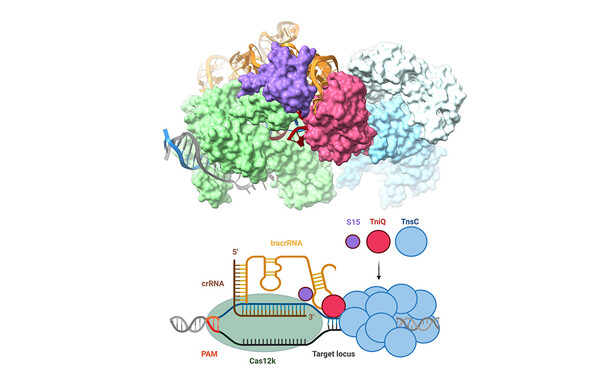
To get a bigger picture of the mechanisms, functions and applications of transposons, we employ an integrative approach, combining structural biology methods with biotechnological approaches. We analyse the macromolecular organization and the mechanistic details underlying DNA mobilization using cryo-electron microscopy and X-ray crystallography together with biochemical and biophysical methods. We investigate the interplay between transposons and host machineries, as CRISPR-Cas systems, and the biotechnological potential of these interactions using cell-based functional assays, protein design and genome engineering experiments. We extend these studies to technologically and therapeutically relevant cells and organisms with collaboration partners to develop transposon-based applications.