Reconstitution of BNIP3/NIX-mitophagy initiation reveals hierarchical flexibility of the autophagy machinery.
2025 Nature cell biology
PMID: 40715440
Adriaenssens Elias, Schaar Stefan, Cook Annan S I, Stuke Jan F M, Sawa-Makarska Justyna, Nguyen Thanh Ngoc, Ren Xuefeng, Schuschnig Martina, Romanov Julia, Khuu Grace, Uoselis Louise, Lazarou Michael, Hummer Gerhard, Hurley James H, Martens Sascha
Recruitment of autophagy initiator TAX1BP1 advances aggrephagy from cargo collection to sequestration.
2024 The EMBO journal;43(23):5910, 5940, 5910-5940.
PMID: 39448883
Bauer Bernd, Idinger Jonas, Schuschnig Martina, Ferrari Luca, Martens Sascha
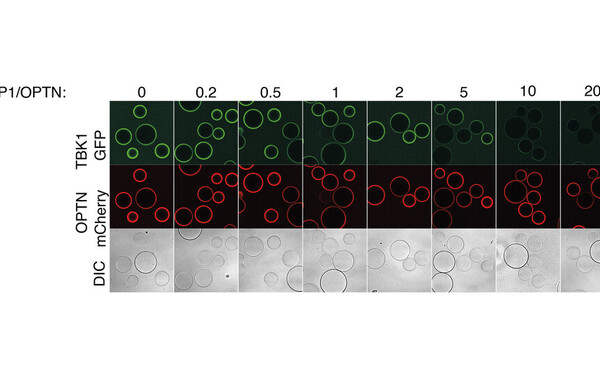
Control of mitophagy initiation and progression by the TBK1 adaptors NAP1 and SINTBAD.
2024 Nature structural & molecular biology;31(11):1717, 1731, 1717-1731.
PMID: 38918639
Adriaenssens Elias, Nguyen Thanh Ngoc, Sawa-Makarska Justyna, Khuu Grace, Schuschnig Martina, Shoebridge Stephen, Skulsuppaisarn Marvin, Watts Emily Maria, Csalyi Kitti Dora, Padman Benjamin Scott, Lazarou Michael, Martens Sascha
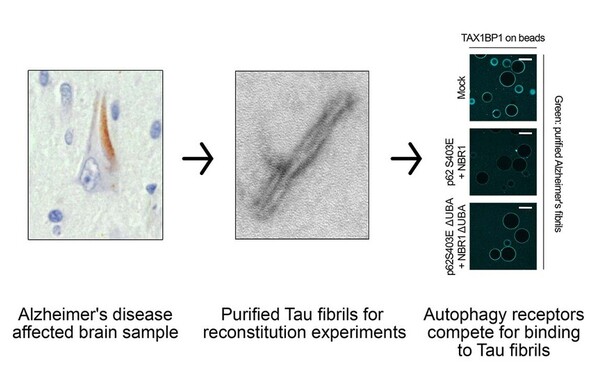
Tau fibrils evade autophagy by excessive p62 coating and TAX1BP1 exclusion.
2024 Science advances;10(24):eadm8449.
PMID: 38865459
Ferrari Luca, Bauer Bernd, Qiu Yue, Schuschnig Martina, Klotz Sigrid, Anrather Dorothea, Juretschke Thomas, Beli Petra, Gelpi Ellen, Martens Sascha
Faa1 membrane binding drives positive feedback in autophagosome biogenesis via fatty acid activation.
2024 The Journal of cell biology;223(7)
PMID: 38573225
Baumann Verena, Achleitner Sonja, Tulli Susanna, Schuschnig Martina, Klune Lara, Martens Sascha
Sequestration of translation initiation factors in p62 condensates.
2023 Cell reports;42(12):113583.
PMID: 38096057
Danieli Alberto, Vucak Georg, Baccarini Manuela, Martens Sascha
Unconventional initiation of PINK1/Parkin mitophagy by Optineurin.
2023 Molecular cell;83(10):1693, 1709.e9, 1693-1709.e9.
PMID: 37207627
Nguyen Thanh Ngoc, Sawa-Makarska Justyna, Khuu Grace, Lam Wai Kit, Adriaenssens Elias, Fracchiolla Dorotea, Shoebridge Stephen, Bernklau Daniel, Padman Benjamin Scott, Skulsuppaisarn Marvin, Lindblom Runa S J, Martens Sascha, Lazarou Michael
Reconstitution defines the roles of p62, NBR1 and TAX1BP1 in ubiquitin condensate formation and autophagy initiation.
2021 Nature communications;12(1):5212.
PMID: 34471133
Turco Eleonora, Savova Adriana, Gere Flora, Ferrari Luca, Romanov Julia, Schuschnig Martina, Martens Sascha
Reconstitution of autophagosome nucleation defines Atg9 vesicles as seeds for membrane formation.
2020 Science (New York, N.Y.);369(6508)
PMID: 32883836
Sawa-Makarska Justyna, Baumann Verena, Coudevylle Nicolas, von Bülow Sören, Nogellova Veronika, Abert Christine, Schuschnig Martina, Graef Martin, Hummer Gerhard, Martens Sascha
A PI3K-WIPI2 positive feedback loop allosterically activates LC3 lipidation in autophagy.
2020 The Journal of cell biology;219(7)
PMID: 32437499
Fracchiolla Dorotea, Chang Chunmei, Hurley James H, Martens Sascha
FIP200 Claw Domain Binding to p62 Promotes Autophagosome Formation at Ubiquitin Condensates.
2019 Molecular cell;74(2):330, 346.e11, 330-346.e11.
PMID: 30853400
Turco Eleonora, Witt Marie, Abert Christine, Bock-Bierbaum Tobias, Su Ming-Yuan, Trapannone Riccardo, Sztacho Martin, Danieli Alberto, Shi Xiaoshan, Zaffagnini Gabriele, Gamper Annamaria, Schuschnig Martina, Fracchiolla Dorotea, Bernklau Daniel, Romanov Julia, Hartl Markus, Hurley James H, Daumke Oliver, Martens Sascha
p62 filaments capture and present ubiquitinated cargos for autophagy.
2018 The EMBO journal;37(5)
PMID: 29343546
Zaffagnini Gabriele, Savova Adriana, Danieli Alberto, Romanov Julia, Tremel Shirley, Ebner Michael, Peterbauer Thomas, Sztacho Martin, Trapannone Riccardo, Tarafder Abul K, Sachse Carsten, Martens Sascha
Mechanism of cargo-directed Atg8 conjugation during selective autophagy.
2016 eLife;5
PMID: 27879200
Fracchiolla Dorotea, Sawa-Makarska Justyna, Zens Bettina, Ruiter Anita de, Zaffagnini Gabriele, Brezovich Andrea, Romanov Julia, Runggatscher Kathrin, Kraft Claudine, Zagrovic Bojan, Martens Sascha
Oligomerization of p62 allows for selection of ubiquitinated cargo and isolation membrane during selective autophagy.
2015 eLife;4:e08941.
PMID: 26413874
Wurzer Bettina, Zaffagnini Gabriele, Fracchiolla Dorotea, Turco Eleonora, Abert Christine, Romanov Julia, Martens Sascha
Cargo binding to Atg19 unmasks additional Atg8 binding sites to mediate membrane-cargo apposition during selective autophagy.
2014 Nature cell biology;16(5):425, 433, 425-433.
PMID: 24705553
Sawa-Makarska Justyna, Abert Christine, Romanov Julia, Zens Bettina, Ibiricu Iosune, Martens Sascha
Mechanism and functions of membrane binding by the Atg5-Atg12/Atg16 complex during autophagosome formation.
2012 The EMBO journal;31(22):4304, 4317, 4304-17.
PMID: 23064152
Romanov Julia, Walczak Marta, Ibiricu Iosune, Schüchner Stefan, Ogris Egon, Kraft Claudine, Martens Sascha