Spotlights
Transcription factor STAT1 is a macrophage-activating signal integration platform
Macrophage activation by microbe-induced signals and signals by the IFN-γ receptor (JAK-STAT signaling) converge on transcription factor STAT1. The former move STAT1 to the nucleus whereas the latter modulate its transcription factor activity. Kovarik et al. (1998) EMBO J. 17, 3660-3668. Kovarik et al. PNAS (1999) 96, 13956-13961. Varinou et al. Immunity (2003) 19, 793-802.
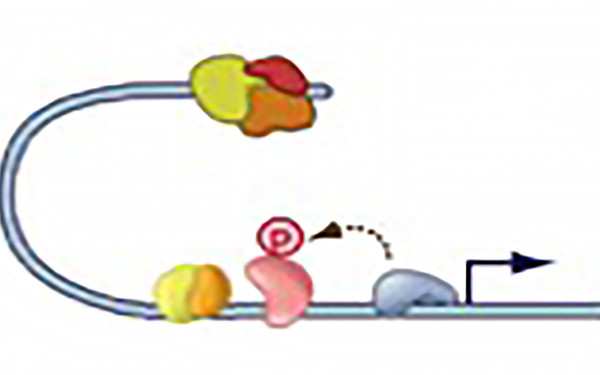
Gene Promoters integrate NFκB and JAK-STAT signaling
Microbial infection causes type I IFN synthesis and JAK-STAT signaling. In addition, the NFκB pathway is activated. The two pathways perform complementary functions in transcriptional initiation such as recruiting the mediator complex and the kinases phosphorylating RNA polymerase. This results in a synergistic effect on gene expression. Farlik et al. (2010) Immunity 33:25-34. Wienerroither et al. (2015) Cell Reports 12: 300-312
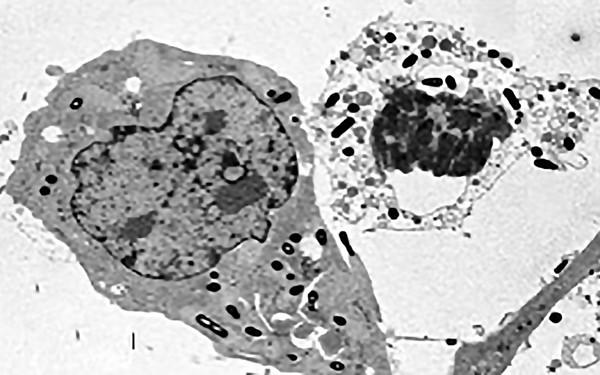
Interferons increase the vulnerability to infection by intracellular bacteria
Type I IFN, produced during infection with the intracellular bacterium Listeria monocytogenes increase the death of infected macrophages. Likewise, mice are more resistant to L. monocytogenes if a type I IFN response is prevented. Stockinger et al. J. Immunol. (2002) 169, 6522-6529. Stockinger et al. PLoS Pathogens (2009) 5(3): e1000355.
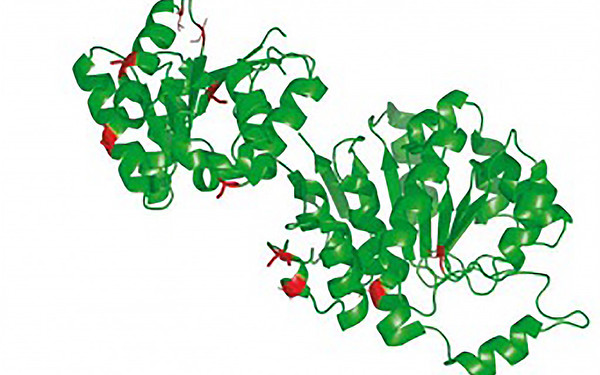
Sex-dependent impact of RNA-helicase DDX3X on immunity against bacteria
The X-chromosome-encoded enzyme provides antibacterial immunity by increasing transcription of antimicrobial genes. Loss of DDX3X is tolerated in the male immune system due to a y-chromosomal homologue, DDX3Y. Female cells have a strongly reduced life span and female mice lacking DDX3X in the immune system die in utero. Soulat et al. EMBO J. (2008) 27:2135-2146. Szappanos et al. PLoS Pathogens (2018) 14(11): e1007397.