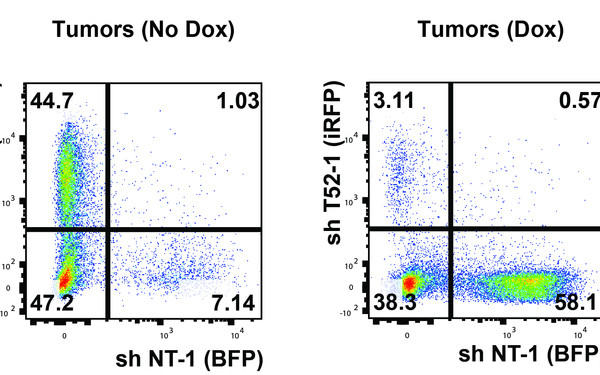
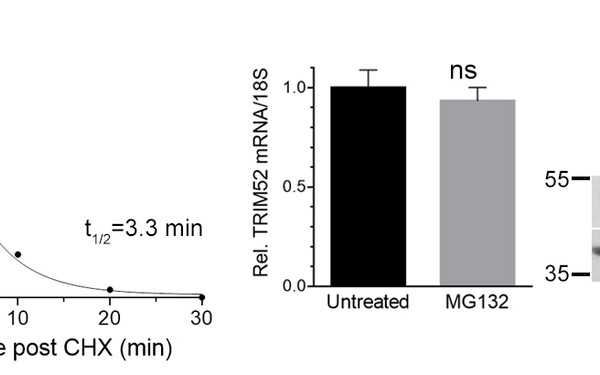
The Question
Strict control over degradation of individual proteins at the correct time and space in cells is key for dynamic transitions in cell signaling. Protein modification by the modifier ubiquitin is recognized as a fundamental mechanism to target proteins to the main protease complex in the cell: the proteasome. Cell-free and structural studies have provided mechanistic insight into how ubiquitinated proteins are recognized and degraded by the proteasome. However, many principles and regulators of how ubiquitination, proteasome targeting, target extraction from sub-cellular sites, and fate-determinations at the proteasome in the crowded intracellular space are achieved, remain unknown.
Main questions addressed are: 1) How are immune- and cancer-associated proteins targeted to the proteasome, 2) which other cellular factors control their degradation, 3) are these processes deregulated in cancer and immune disease, and 4) how do they mechanistically determine cellular protein fate decisions?





 None
None SUWITA Johannes (Pauli), MATZINGER Manuel (Tech Hub), PANDA Aswini (Falk)
SUWITA Johannes (Pauli), MATZINGER Manuel (Tech Hub), PANDA Aswini (Falk)