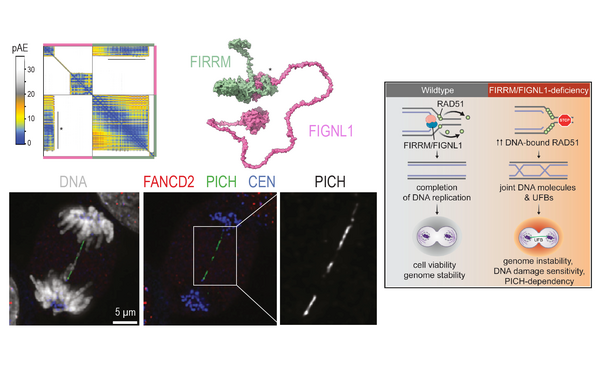
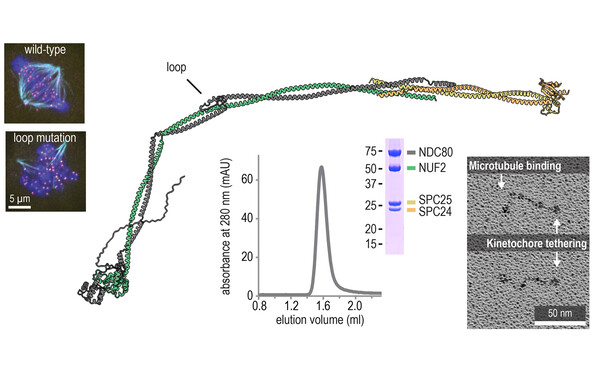
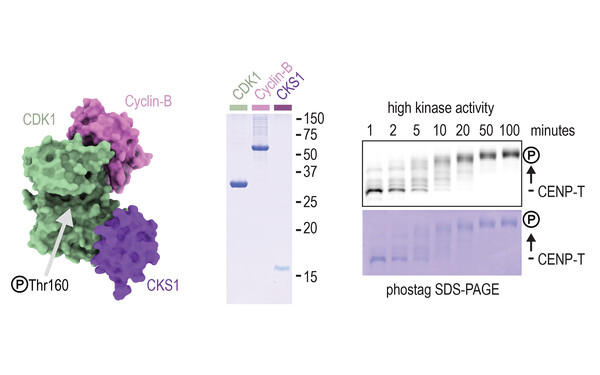
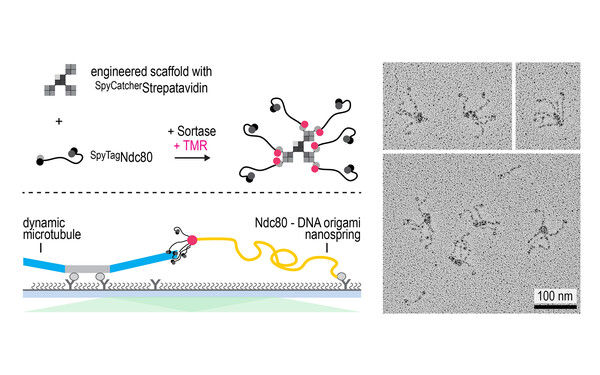
Pim Huis in 't Veld
Pim studied Molecular Life Sciences in Nijmegen, the Netherlands. He moved to Vienna in 2009 to study sister chromatid cohesion in the lab of Jan-Michael Peters at the IMP (PhD). In 2015, he joined the lab of Andrea Musacchio (MPI-MOPH, Germany) to investigate how kinetochores attach to the mitotic spindle. In August 2023, Pim started as a group leader at the Max Perutz Labs.