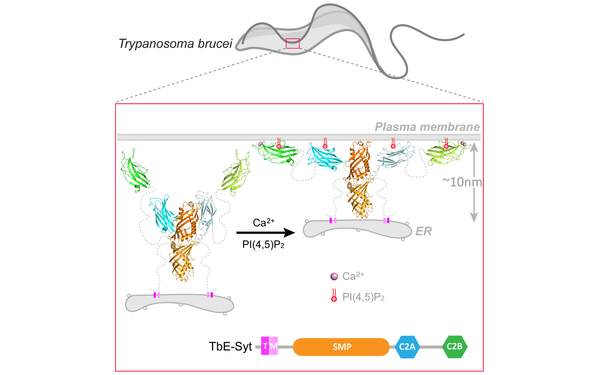
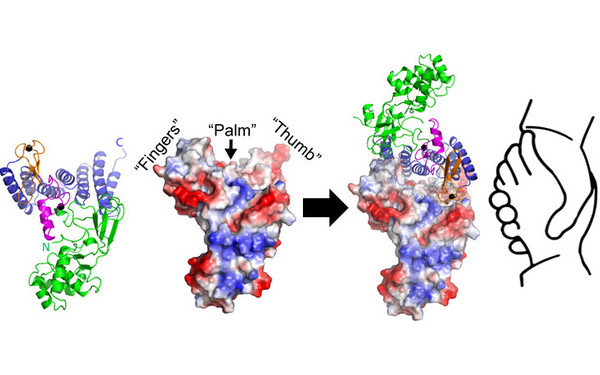
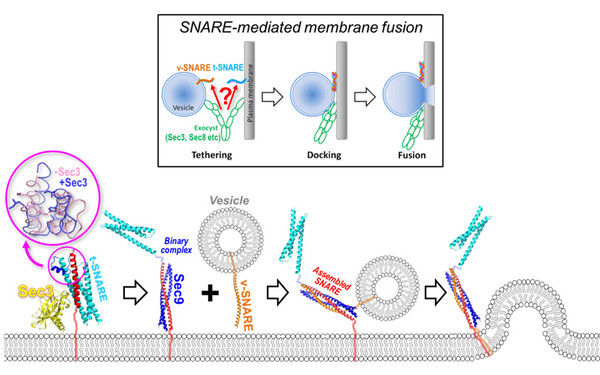
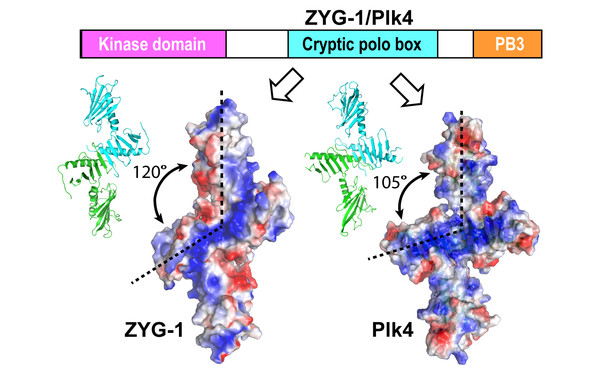
Our International Joint Project "CytoPocket" has been approved for funding!
Project title: "Flagellar pocket membrane-Cystoskeleton dyanmics in T. brucei" (PIN2972425)
Our collaborative grant application with Dr. Melanie Bonhivers and Dr. Laure Béven of the University of Bordeaux, France, has been successful. The project has been awarded a total of €970,835.88 through the "Principal Investigator Projects International" program by both the FWF (Austria) and the ANR (France). This grant will support our joint research into flagellar pocket membrane-cytoskeleton dynamics in the human parasite Trypanosoma brucei.
Duration: 01 April, 2026 - 31 March, 2030.
International Joint Project (FWF-ANR)
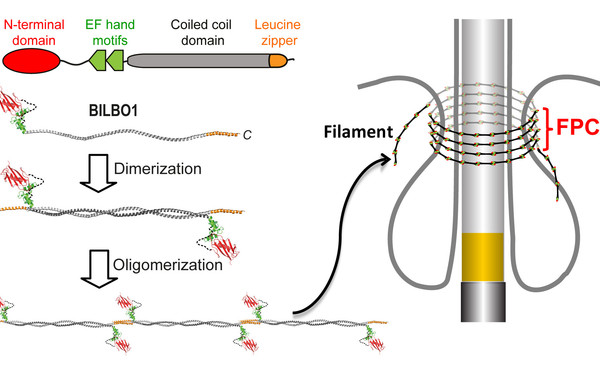
Project title: "Why and how trypanosomes build a flagellar pocket collar" (I 4960-B)
In collaborataion with Dr. Melanie Bonhivers from the University of Bordeaux and co-funded by the FWF (Austria) and the ANR (France). The grant (Sum: 705,485.70 EUR) will support our joint effort to study the flagellar pocket collar (FPC) of the human parasite Trypanosoma brucei. We aim to explain how a new FPC is formed, why it is essential, what polymers/proteins are needed, and how they interact.
Duration: 01 Oct, 2020 - 31 Mar, 2026.
Austrian Scientific Fund (FWF) - Collaborative project
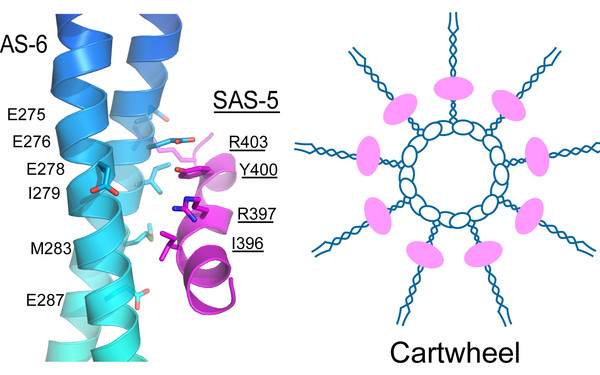
Project title: "Structural basis of tRNA synthetase-based selfish killer" (P 34880)
This grant (Sum: 603,960.00 EUR) will support our collaborative work with Dr. Alejandro Burga’s group (IMBA, VBC) on eukayrotic Selfish Elements, which underlie numerous cases of hybrid dysgenesis, sterility and genetic incompatibilities in nature. We will employ our complementary expertise in genetics and structural biology to dissect the molecular mechanism of a set of selfish elements in the nematodes C. elegans and C. tropicalis.
Duration: 01 July, 2021 - 30 June, 2026.
American National Institutes of Health NIH-R01 (collaborative)
Project title: "Mechanism of infectivity acquisition in African trypanosomes" (2 R01 AI110325-06)
A newly approved NIH-R01 grant ($345,724.00; total: $2,311,805.00) to support our collaborative work with Prof. Christian Tschudi's group at the Yale School of Medicine to unveil the molecular mechanism of the infectivity acquisition in African Trypanosomes. Our complementary expertise will provide unique opportunities for us to illuminate the developmental program leading from non-infective procyclics to infectious metacyclics, a crucial process in the T. brucei life cycle.
Duration: 01 Mar, 2019 - 29 Feb, 2024.
International Doctoral Program "Integrative Structural Biology"
The Group Dong participated in and was one of the seven full members of the Doctoral Program "Integrative Structural Biology" (W-1258), which was reviewed and funded by the Austrian Science Fund FWF.
Duration: 01 Jan, 2016 - 30 June, 2020.
Austrian Scientific Fund (FWF) - Stand alone project
Project title: “Structural Characterization of ZYG-1 in Centriole Assembly" (P 28231)”
Duration: 01 July, 2015 - 30 June, 2020.
Austrian Scientific Fund (FWF) - Stand alone project
Project title: “Structural studies of the Trypanosoma brucei protein TbBILBO1" (P 24383-B21)
Duration: 01 May, 2012 - 30 Apr, 2016.
Austrian Scientific Fund (FWF) - Stand alone project
Project title: “Structural Studies of the Intraflagellar Transport Complexes" (P 23440-B20)
Duration: 01 Apr, 2011 - 31 Oct, 2015.
WWTF - Molecular Mechanisms and Methods
Project title: "Towards sustainable food and bioenergy security for society: Establishing an academic compound screening platform in Vienna to characterize and modulate Strigolactone synthesis in plants" (WWTF 2009) Role: Research Partner (PI: Dr. T. Sieberer). Duration: 2011 - 2014.