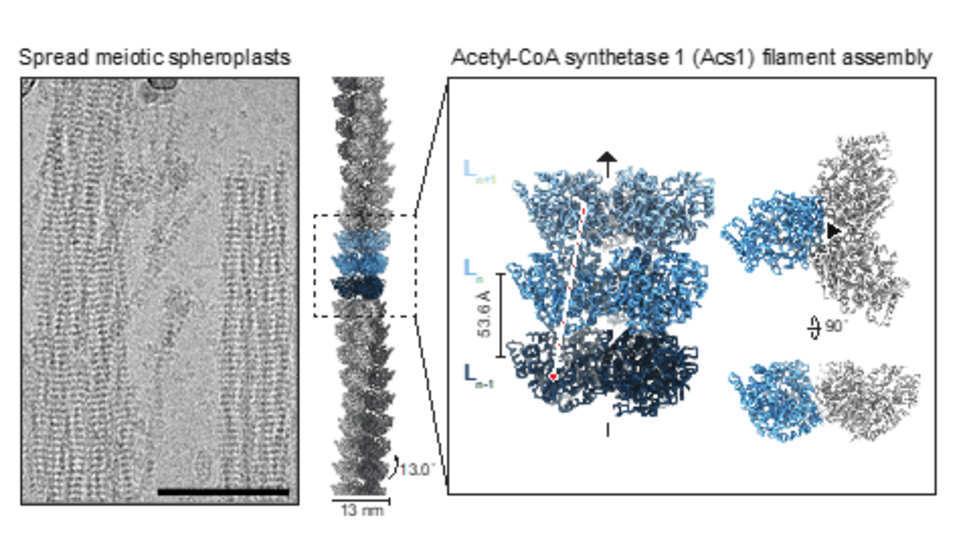

Meiosis, a specialized cell division essential for sexual reproduction, produces four haploid spores in the baker's yeast, Saccharomyces cerevisiae. While imaging meiotic cells, Jannik Hugener, a joint PhD student in the Matos and Pilhofer labs, discovered filaments of unknown composition and function decorating their cytoplasms, nuclei and mitochondria. Group leader Joao Matos explains: “These filaments appeared in cells undergoing meiosis. Spores, the products of meiosis in yeast, may need to survive for years, entering a dormant state and proliferating again only when conditions improve. These filaments seem to aid in their recovery.” By employing cryo-electron tomography and single-particle cryo-electron microscopy, the team was able to reveal both the protein composition and the repetitive nature of the filaments.
Whereas mitochondrial filaments are composed of the conserved aldehyde dehydrogenase 4 (Ald4), the nuclear and cytoplasmic filaments are composed of acetyl-CoA-synthetase 1 (Acs1). Acs1 generates acetyl-CoA, a key metabolic intermediate that links glycolysis with the citric acid cycle. In yeast, acetyl-CoA is also crucial for histone acetylation, a process that regulates DNA packaging and gene expression. Acetyl-CoA synthesis involves a two-step reaction using ATP as the energy source. Joao explains, "Our experiments indicate the presence of acetyl-AMP bound to filamentous Acs1. This suggests that the enzyme completes the first step of the reaction but cannot proceed to the second." He adds, "Acs1 expends energy to form a reaction intermediate, but structural constraints prevent it from completing the process. We propose that Acs1 produces acetyl-CoA at the onset of meiosis, then inhibits itself by forming filaments. Acs1 filaments are then inherited by spores. Filament disassembly is then used to generate acetyl-CoA without the need for ATP, enabling quick re-entry into cell proliferation.”
The researchers found that Acs1 filaments are dispensable for acetyl-CoA synthesis during meiosis but are crucial for spore recovery after extended dormancy. Joao explains, "Cells appear to form filamentous structures to endure prolonged periods of unfavorable conditions. When the meiotic products inherit Acs1 filaments, they can produce acetyl-CoA without expending ATP." When conditions become favorable, cells germinate and resume growth, even after weeks of dormancy. The researchers’ experiments indicate that the longer the dormancy period, the more essential Acs1 filaments become for successful return to proliferation.
To study the filaments of unknow composition in the first place, the Matos lab and their collaborators developed a workflow termed ‘FilamentID,’ which integrates multiscale cryo-electron tomography and microscopy. Joao emphasizes that the method should be applicable to address numerous biological questions: “FilamentID can be broadly used to determine the composition of uncharacterized filamentous assemblies.” In the future, the Matos lab plans to investigate other types of dormant cells and determine if filament formation is a conserved feature of the human orthologs of Acs1 and Ald4.