The Question
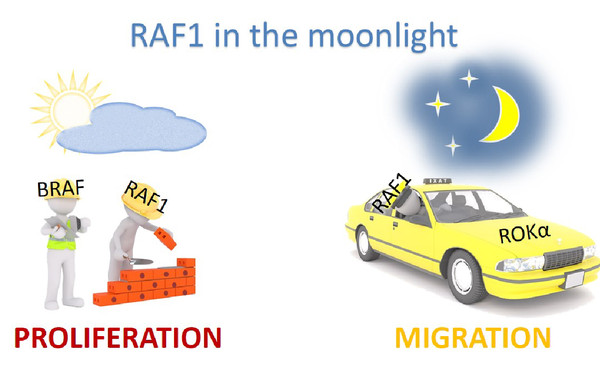
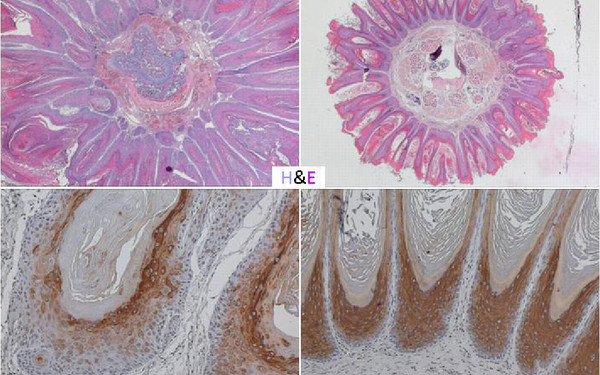
Cells survive, proliferate, and differentiate by interpreting the signals from the environment and translating them into the right output. If signaling goes awry, even in a few cells, the whole organism is at risk. We focus on signaling by the RAF/MEK/ERK pathway, an evolutionarily conserved kinase module which relays signals through consecutive phosphorylation of proteins arranged in three tiers. Constitutive activation of the pathway is a key event in several human malignancies and developmental disorders. Thus, proteins in this pathway are attractive therapeutic targets.
Strikingly, the pathway increases in complexity with evolution - from one paralog in simple organisms to three RAF, two MEK and two ERK paralogs in vertebrates. We investigate the essential roles of RAF and MEK paralogs and have discovered that they are indispensable for pathway cross‐talk, with functional consequences for homeostasis at the molecular, cellular, and organismal level.