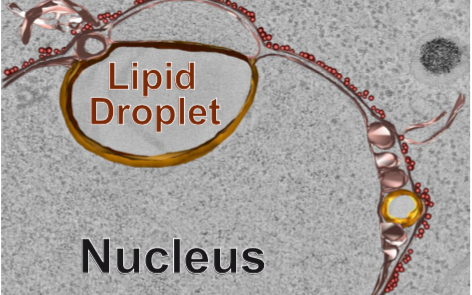

The cell nucleus is a fascinating organelle, in which an organism’s DNA is protected, decoded and duplicated. The nucleus is surrounded by not one, but two membrane sheets: the outer and the inner nuclear membrane. These two membranes connect with each other at membrane openings occupied by nuclear pores. The outer nuclear membrane also connects to the endoplasmic reticulum (ER), an extended membrane network in the cytoplasm. From a cytoplasmic view, the inner nuclear membrane is the most remote territory of the ER, both in distance and difficulty of access. To get there, any lipid or protein must pass through the nuclear pores. Whereas the ER and outer nuclear membrane are highly active in converting nutrients into building blocks for lipids and membranes, the inner nuclear membrane was thought to be metabolically inactive and to receive its entire lipids from the ER via the nuclear pore route.
A team of two, Anete Romanauska and Alwin Köhler, now report in Cell new findings that overturn this perception of the inner nuclear membrane as a dark backyard of the ER. They discovered metabolic turnover of lipids at the inner nuclear membrane of budding yeast and found that the inner nuclear membrane can form lipid droplets that are used for lipid storage. These droplets can occupy a huge portion of nuclear space and communicate with the inner nuclear membrane through specialized membrane bridges. A high-fat diet is enough for these droplets to form. The authors identify the genetic circuit for the synthesis of nuclear lipid droplets and show how these organelles participate in gene regulation. Further, they identify a factor that is required for the proper exchange of lipids between the droplets and the inner nuclear membrane. The human counterpart of this factor is mutated in a form of congenital lipodystrophy, a condition characterized by a drastic loss of body fat.
Romanauska and Köhler also find evidence that the inner nuclear membrane is different in its lipid composition from the outer nuclear membrane and the ER. Hence, by no means is the inner nuclear membrane a simple extension of these other membrane systems. It has a unique lipid functionality, that Romanauska and Köhler now put a spotlight on. Looking ahead, this study opens exciting avenues for exploring how inner nuclear membrane lipids affect the genome and its regulation. Moreover, it will be exciting to learn, which role the inner nuclear membrane plays in congenital lipodystrophy and in other human metabolic diseases.
Getting to this point was not easy. “Tool-building was a key part of this study,” says Anete Romanauska, who started the project in her Master’s thesis and is now in the second year of her PhD studies in Alwin Köhler’s lab. “We created a set of genetically encoded, fluorescent lipid biosensors that allowed us to see the lipids inside the nucleus,” Anete explains. Both Anete and Alwin vividly remember the moment when they first saw nuclear lipid droplets glowing inside the nucleus.” Alwin adds: “I truly admire Anete’s boundless energy and tenacity. Most inspiring for me was the constant refinement of our hypotheses by bouncing ideas back and forth. I typically get excited quickly and Anete tells me to calm down. This combination worked quite well.” What’s in the spotlight next? The most pressing questions, Alwin and Anete agree, center on the cellular function of nuclear lipid droplets. “I have some new data,” says Anete.
Publication in Cell
Anete Romanauska, Alwin Köhler: The inner nuclear membrane is a metabolically active territory that generates nuclear lipid droplets.