

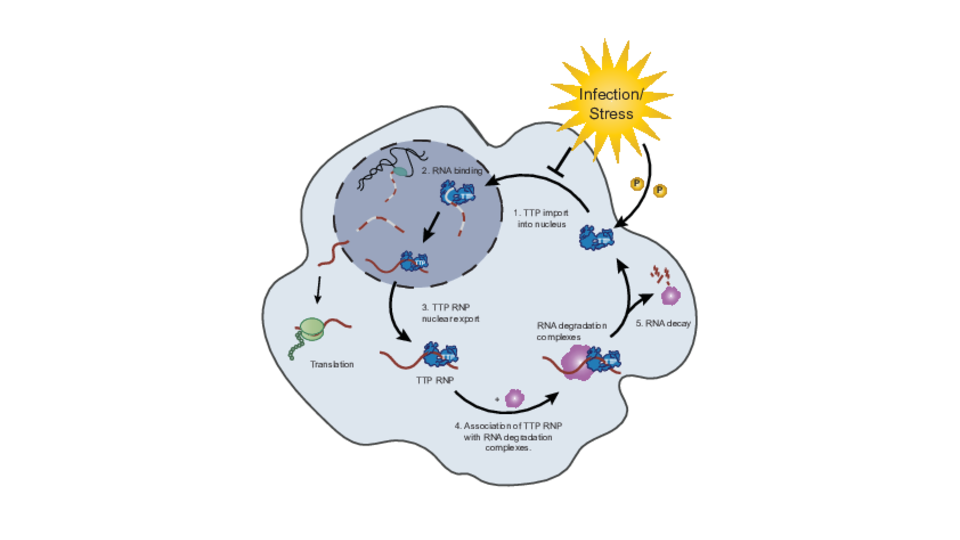
The anti-inflammatory RNA-binding protein tristetraprolin (TTP) plays a crucial role in controlling inflammatory gene expression, as its deletion results in spontaneous lethal multi-organ inflammation. However, the precise mechanisms by which TTP helps regulate inflammation have remained unclear – until now. In the Kovarik lab’s new study, first author Annika Bestehorn reveals: “We discovered a mechanism whereby TTP regulates RNA decay at the pre-mRNA stage in the nucleus, rather than in the cytoplasm where canonical RNA decay processes occur. This way, the target mRNAs – those containing AU-rich elements – are licensed for degradation before translation.”
The nuclear transport of TTP is essential for its role in RNA degradation. “Our work uncovers a new layer of regulation, in which the shuttling of TTP between the nucleus and cytoplasm determines the fate of specific mRNAs before they are even translated”, Annika elaborates. Whereas the fate decision – resolving inflammation – is executed in the nucleus, RNA degradation takes place in the cytoplasm following the export of TTP-bound pre-mRNA complexes.
Group leader Pavel Kovarik highlights the wider implications of their study: “The nuclear licensing mechanism is likely relevant also for biological processes beyond inflammation. A major conceptual advance of our study is the discovery that the degradation of mRNAs that code for inflammatory mediators is a regulated, not stochastic process.”
The study involved contributions from the Versteeg and Baccarini labs and support from the Perutz Mass Spectrometry facility. Ten PhD students from the FWF-funded SMICH Doctoral School – led by Perutz group leader Martin Leeb – contributed as authors, reflecting the strong sense of community and collaboration at the institute.
DOI: 10.1016/j.molcel.2025.01.001