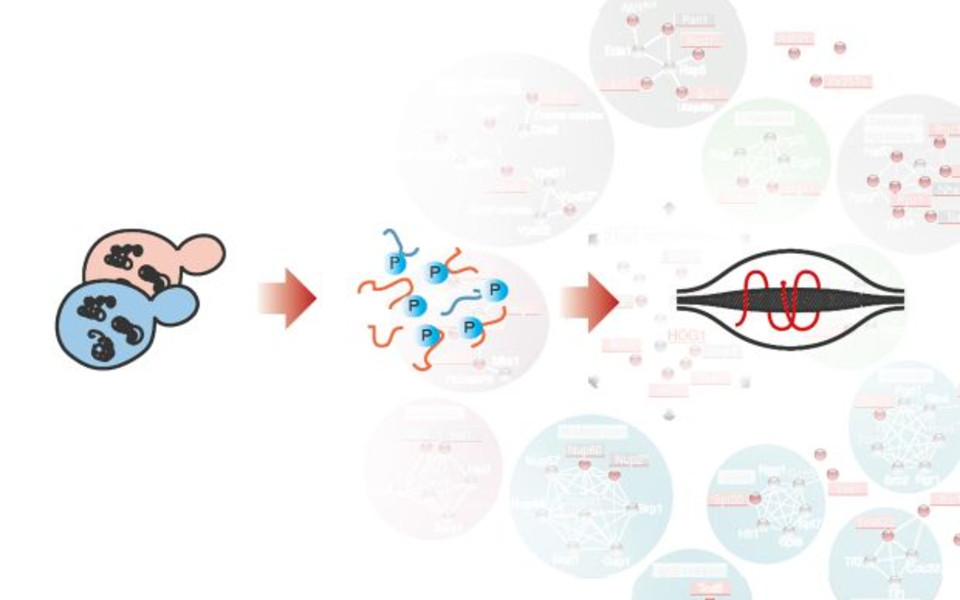
Adaptation processes are usually facilitated by complex intracellular signal transduction systems that can be imagined as hierarchical cascades of proteins, in which a signal is transmitted from the cell’s surface to intracellular effector molecules. An example for an extreme environmental condition is high extracellular osmolarity, which disrupts the balance between molecules located inside and outside of a cell. For the budding yeast Saccharomyces cerevisiae such hyperosmotic stress means rapid water loss and shrinkage. Yeast cells respond to the stressor with a temporary cell-cycle arrest, reprogramming of gene expression patterns and an increased intracellular concentration of the osmolyte glycerol.
These changes are to a large extent mediated by the “high osmolarity glycerol (HOG)” pathway, a close relative of one of the mitogen-activated protein kinase (MAPK) pathways in mammals. The heart of the HOG pathway is the protein kinase Hog1. In order to regulate its target proteins, Hog1 adds phosphate moieties in a process called “phosphorylation”. In a recent study published in the prestigious journal “Science Signaling”, Wolfgang Reiter and his team from Gustav Ammerer’s group at the Max F. Perutz Laboratories (MFPL) now report the identification of a closely defined set of at least 25 direct target proteins of Hog1, next to a broad set of indirect targets. The target proteins were identified by mass spectrometry analysis, a technique that measures the masses of peptides within a sample and thus enables researchers to detect even the slightest modifications of amino acids. To validate the interaction partners of Hog1, the group used an assay that measures the physical proximity of proteins.
On top of these findings, the group reports another player in the osmostress response, the protein kinase Rck2, which is a direct target of Hog1. Rck2 seems to be a central hub for phosphorylation events mediated by Hog1, meaning that it controls indirect Hog1 signaling in a complex manner. Wolfgang Reiter hopes that these new insights will serve as a basis for other scientists, who wish to dig deeper into the complex connections of the HOG pathway and possibly also of MAPK signaling in general. Many possibilities for the conduct of future studies arise, thanks to the identification of such a broad set of new factors and the innovative study design.
Publication in Science Signaling:
Natalie Romanov, David Maria Hollenstein, Marion Janschitz, Gustav Ammerer, Dorothea Anrather and Wolfgang Reiter. Identifying protein kinase-specific effectors of the osmostress response in yeast. Science Signaling, DOI: 10.1126/scisignal.aag2435