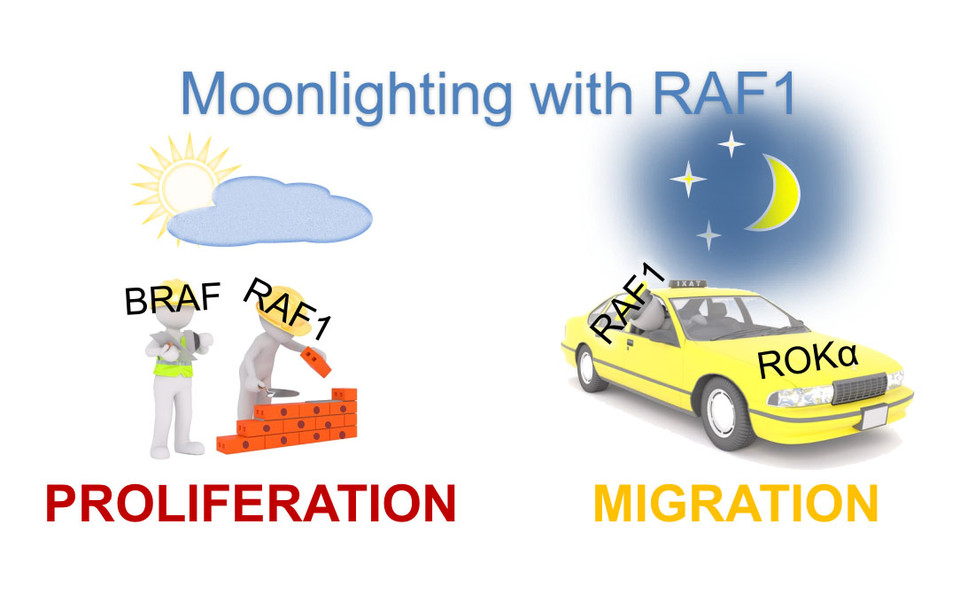
Oftentimes, these cascades feature families of proteins that can carry out similar functions. The RAF kinases, which are involved in the control of key cellular pathways such as proliferation and migration, are members of such a family. In their most recent Science Signaling publication, Manuela Baccarini and her team were now able to identify that RAF1, a member of the RAF family , acts as a signalling hub linking proliferation to cell shape, adhesion and motility. Both proliferation and migration are deregulated in human diseases including cancer. Identification of one protein that has implications in both processes is thus highly exciting. Specifically, RAF1 activation by growth factors and oncogenes generates an array of RAF1 species characterized by distinct modification sites, called phosphorylation patterns. Much as in a blind date, these patterns identify RAF1 as an interaction partner for proteins working in signalling pathways, leading to cell proliferation or to changes in the “skeleton” of the cell and in cell shape and migration.
In an unexpected twist, the Baccarini lab showed that, in order to be a good partner for the proteins that regulate cell shape and migration, RAF1 must already have encountered the proteins that regulate proliferation. “RAF1 can be likened to a multiple job holder: it has a daytime job in proliferation cascades and “moonlights” as a regulator of the cytoskeleton. Strikingly, RAF1 has multiple essential functions in cancer development, and these functions are associated with its moonlight, not its daytime job. Therefore, the “moonlighting” function of RAF1 might explain why it was retained throughout evolution.”, says Manuela Baccarini.
The experimental results could be recapitulated in a complex, custom-made mathematical model. This is an entirely new area for the Baccarini group, made possible thanks to a collaboration with the group of Tomek Lipniacki at the Institute of Fundamental Technological Research in Warsaw, Poland. The collaboration was funded by the WWTF. Baccarini and Lipniacki hope that this and similar models will enable other researchers to make predictions about how phosphorylation patterns generated during activation influence the biological outcome of a signal.
Publication in Science Signaling:
Andrea Varga, Karin Ehrenreiter, Bertram Aschenbrenner, Pawel Kocieniewski, Marek Kochanczyk, Tomasz Lipniacki and Manuela Baccarini: RAF1/BRAF dimerization integrates the signal from RAS to ERK and ROKa. Science Signaling, DOI: 10.1126/scisignal.aai8482