

The ability to regenerate – from individual cell types to entire organs or complex tissues – is crucial for all living species. Annelid worms like Platynereis dumerilii can regenerate parts of their posterior body after injury, but the molecular mechanisms driving this process were poorly understood. Co-first author Leonie Adelmann says: “The worm’s regeneration ability is naturally modulated – it regenerates rapidly in its immature state but loses this capacity upon sexual maturation. This switch offers unique insights into how regeneration is regulated.” Normally, the so-called segment-addition zone (SAZ), located after the last segment, buds off new segments during growth. After an injury, although the SAZ is lost, it regenerates swiftly, allowing the worm to quickly restore its missing segments.
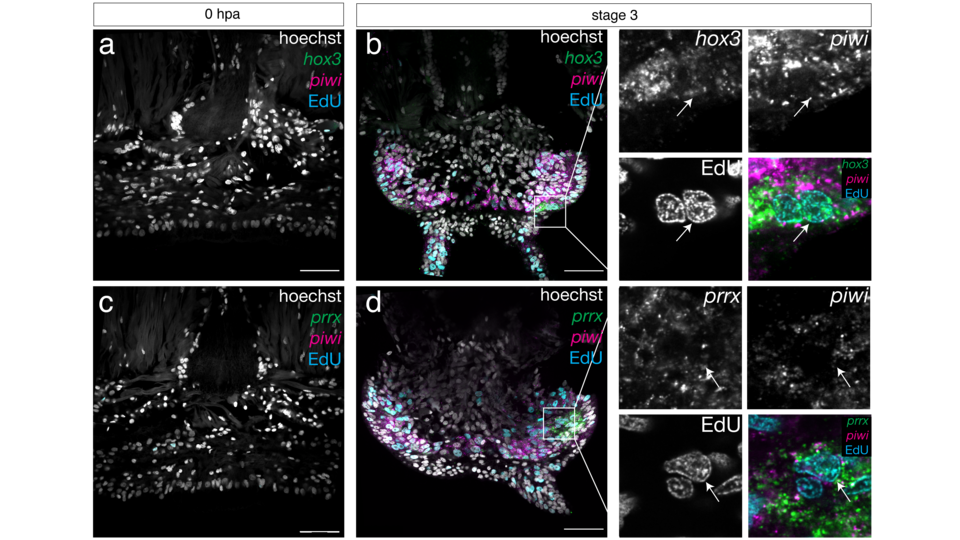
Leonie, Alexander and the Raible team reveal how the lost SAZ is rebuilt and uncover the molecular mechanisms behind this process. Unlike other species, regeneration in the marine worm does not rely on a pool of stem cells. Instead, specialized cells undergo de-differentiation, reverting to stem cell-like states after injury to rebuild the SAZ and form new segments. The researchers found that gene expression in these newly formed stem cells is indeed distinct from their precursor cells. Perutz Group leader Florian Raible elaborates: “The concept of de-differentiation was suggested over 60 years ago, but researchers then lacked the tools to test this idea. Now, we’ve developed tools to understand de-differentiation at a molecular level, providing a foundation for future studies.”
The researchers’ approach of combining single-cell RNA sequencing and transgenesis experiments provides a novel dataset for studying tissue regeneration. “Single-cell transcriptomics allows us to identify cell types and their states, revealing how they respond to injury at the individual level. In our study, we combined this technique with transgenesis to trace stem cell precursors and their progeny. This revealed at least two distinct stem cell populations – one that regenerates tissues like epidermis and neurons, and another one that forms muscles and connective tissue”, explains co-first author Alexander Stockinger. With bioinformatics expertise from Martin Fahrenberger (von Haeseler lab), the team identified molecular markers that designate the stepwise de-differentiation of distinct cells. The collaborating Balavoine lab in Paris performed the transgenesis experiments.
DOI: 10.1038/s41467-024-54041-3
About the Raible lab