
The three-dimensional organization of chromatin plays a crucial role in regulating gene activity. While the euchromatic compartment in the nuclear interior canonically contains active gene loci, the nuclear periphery is typically associated with inactive heterochromatin. The nuclear lamina serves as an anchoring point, where lamin proteins interact with lamina-associated domains (LADs) to tether chromatin to the nuclear membrane. However, some highly active genes, such as MyoD1 - a master regulator of myogenesis in proliferating myoblasts - are also found at the nuclear periphery. Investigating the mechanism that retains active gene loci in this peripheral region was the focus of the Foisner lab’s recent research.
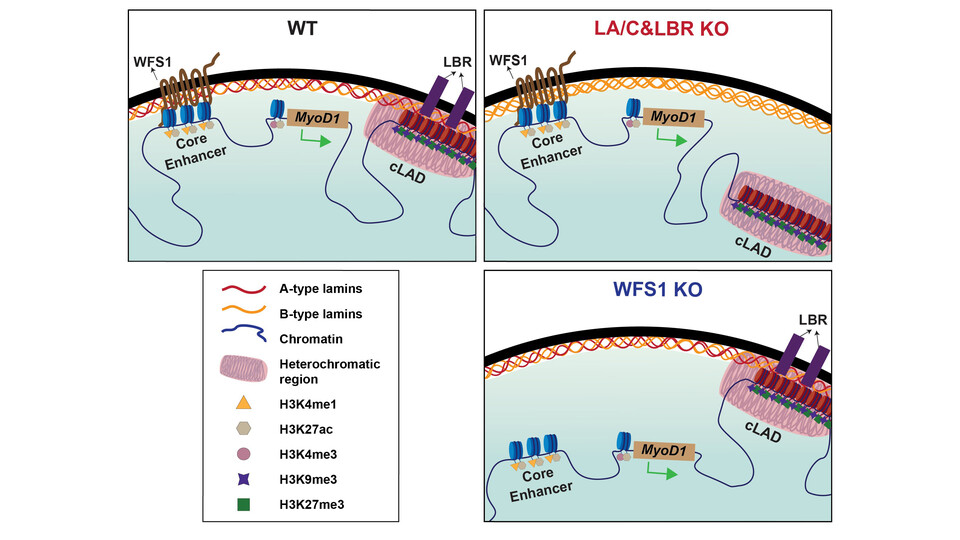
To investigate how MyoD1 is tethered to the nuclear periphery, the Foisner lab developed a novel reporter cell line alongside a pipeline for semi-automated image acquisition and analysis. By tracking its intranuclear position, the scientists could reveal that MyoD1’s localization is independent of the positioning of LADs, but dependent on the transmembrane proteins Tmem38a and WFS1. Tmem38a and WFS1 are, intriguingly, ion channels, but with a previously unappreciated tethering role. “Our findings demonstrate that Tmem38a and WFS1 play a crucial role in anchoring active genes in their peripheral position,” explains group leader Roland Foisner. “Notably, we identified the ER-associated protein WFS1 as a specific tether for MyoD1, marking the first observation of this protein within the nucleus.”
While studying the MyoD1-WFS1 interaction, the Foisner lab uncovered a novel tethering mechanism. Instead of directly binding to the gene itself, WFS1 associates with active enhancer-type cis-regulatory elements at the nuclear periphery, even within the predominantly repressive heterochromatic environment of this region. Roland highlights the significance of their findings: “This discovery underscores that active genes can be actively tethered to the periphery through a protein-driven mechanism, rather than being passively retained there.” This study provides exciting new insights into the intricate organization of chromatin within the cell’s nucleus and implies that intricate three-dimensional positioning may regulate the expression of many more genes than is currently appreciated.
DOI: https://doi.org/10.1038/s41467-025-57758-x
About the Foisner lab